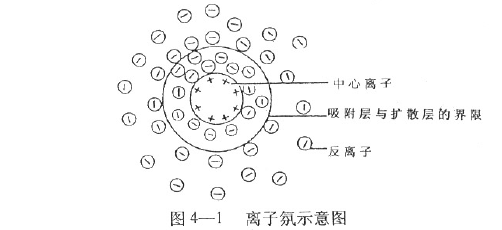
First, the basic concept At present, electrophoresis technology has been widely used for the separation and identification of proteins, nucleic acids and amino acids. What is electrophoresis? In a solution, the phenomenon that charged particles move toward the opposite electrode under the action of an applied electric field is called electrophoresis. Different charged particles travel differently in the same electric field during electrophoresis. The electrophoresis speed is usually expressed by the mobility (M). The migration rate is also called mobility. It is equal to the ratio of the moving velocity (V) to the electric field strength (E) (1), that is, the swimming velocity of the charged particles at the unit electric field strength is called the migration rate. In the formula (2), L is the migration distance and t is the energization time; in Formula 3, V is the terminal voltage applied to both ends of the support, and d is the effective length of the support. Substituting Equations 2 and 3 into Equation 1 yields: As can be seen from Equation 4, when the values ​​of d, L, V, and t are known (measured experimentally), the value of the migration rate M can be calculated. Second, factors affecting the mobility rate According to the physics principle, the electric field force (F) of a charged particle in an electric field is equal to the product of the electric field strength and the amount of electricity charged by the particle. F=EQ In the above formula, E is the electric field strength, and Q is the amount of electricity carried by the particles. According to Stokes' law, when a spherical molecule moves in a solution, the resistance (F/) is proportional to the radius of the spherical molecule (r), the viscosity of the solution (η), and the velocity of migration (v). It is 6π. which is: F/=6πrηv When the electric field force F of the charged particles is equal to the resistance F/, that is, F=F/, EQ = 6πrηv When both ends of the formula are divided by 6πrηE, It can be seen from the above formula that the migration rate is proportional to the charge amount of the spherical molecule, and inversely proportional to the size of the spherical molecule and the viscosity of the medium. In addition to the above properties and the viscosity of the medium, the migration rate is also affected by other external factors. 1. Effect of pH value of electrophoretic medium For amphiphilic molecules such as proteins and amino acids, the pH of the electrophoretic medium determines the nature and amount of the net charge they carry. The pH value is less than the isoelectric point, the molecule has a positive charge and moves toward the negative electrode. If the pH value is greater than the isoelectric point, the molecule has a negative charge and moves toward the positive electrode. The farther the pH deviates from the isoelectric point, the more net charge the molecule carries, and the faster it moves. When the pH of the buffer is equal to its isoelectric point, the molecule is in an isoelectric state and does not move. Since the isoelectric point of serum protein is mostly between pH 4-6, the serum protein is usually separated by a barbital buffer of pH 8.6 or a buffer of Tris. 2. Ionic strength of the buffer Ionic strength is a measure of the amount of charge in a solution. The ionic strength is equal to half the sum of the product of the various ions in the solution and the square of its valence I = 1/2 Σm iZ i 2 The molar concentration of the mi-type ions in the formula, and Zi is the valence of the corresponding ion. Example 1 The ionic strength of two monovalent ionic compounds (such as NaCl) is numerically equal to its molar concentration, such as the ionic strength of a 0.05 mol/L NaCl solution. I = 1/2 (0.05 × 12 + 0.05 × 12) = 0.05 Example 2 The ionic strength of two divalent compounds (CuSO4) is numerically equal to 4 times its molar concentration, for example, the ionic strength of a 0.05 mol/L CuSO4 solution. I = 1/2 (0.05×12 +0.05×12)=0.20 The greater the ion concentration in the solution, or the higher the valence of the ions, the greater the ionic strength. For the buffer, the ionic strength is too low, mainly affecting the buffer capacity of the buffer, and it is difficult to maintain the pH of the medium constant; the ionic strength is too high, and the electrophoresis speed of the charged particles is slowed down. This is because the counter-charged ions in the charged biomacromolecule adsorption solution (Figure 4-1) form a counter-ion atmosphere, just like the atmosphere surrounds the Earth. The closer the center ion is, the greater the counter ion density; conversely, the lower the density. The counter ion layer can be divided into an adsorption layer and a diffusion layer depending on the degree of tightness of the combination of the counter ion and the central ion. Under the action of the electric field, the counter ions of the adsorption layer move along with the central ions. The greater the ionic strength, the more counter ions are in the adsorption layer, and the less the net charge of the moving particle group, the slower the migration speed. 3. Electroosmosis In an electric field, the relative movement of a liquid to a solid support is called electroosmosis. Electroosmosis is caused by the charge on the support. The charge on the support causes the water in the medium to induce an opposite charge. For example, the filter paper cellulose used for electrophoresis on paper has a negative charge; in agar electrophoresis, the agar used has a negative charge due to the presence of a large amount of sulfate, which causes water to induce hydronium ions (H+3O). Under the action of an external electric field, water moves toward the negative electrode. If the sample being measured is also positively charged, the movement is faster; if the sample being measured is negatively charged, the movement is slowed down. Therefore, when electrophoresis, the speed of particle migration is determined by the mobility of the particles themselves and the electroosmotic effect of the solution. Therefore, when using the support, the substance with high electroosmosis should be avoided as much as possible. 4, the impact of electric field strength The electric field strength increases, the electric field force of the charged particles increases, and the swimming speed increases, but the migration rate does not change. As the electric field strength increases, the current intensity increases and the heat generation increases. The adverse consequences of heat production are: (1) causing evaporation of water, changing the pH and ionic strength of the solution; (2) causing an increase in the temperature of the medium to denature the protein. Therefore, the electrophoresis must have a control voltage within a certain range. When performing high-pressure electrophoresis, an effective cooling system must be equipped. Shanghai Chuangsai Technology has excellent performance, interleukin cytokines, fetal bovine serum, electrophoresis equipment scientific instruments, raw material drug standards, chemical reagents, cell culture consumables, Shanghai Chuangsai, mass products special promotions, welcome to inquire! hot air brush was created to replace both blow dryers and wooden hair brushes. Now, instead of holding the devices in both hands, a woman can use a lightweight device that dries and curls (or straightens) hair simultaneously. Hot Air Brush,Hot Hair Brush,Ceramic Hair Brush,Electric Hair Brush Ningbo Meirou Electric Appliance Co.,Ltd. , https://www.mrhaircurler.com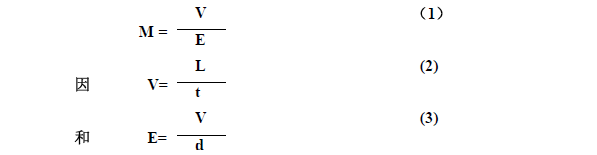
![]()
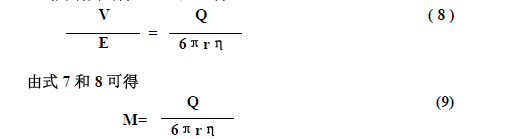
interesting option is a hot air brush with styling attachments. Combo kits may feature several round brushes, attachments for pin-point styling, and concentrators.