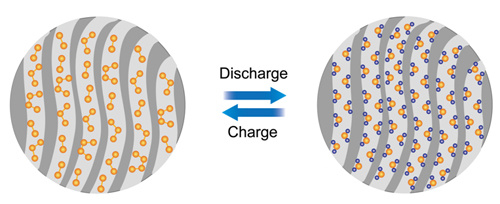
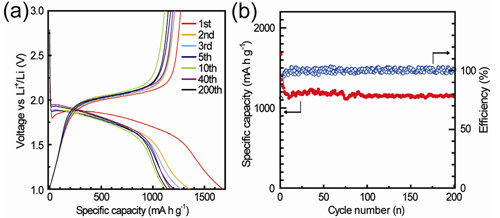
With the support of the National Natural Science Foundation of China, the Ministry of Science and Technology and the Chinese Academy of Sciences, researchers at the Key Laboratory of Molecular Nanostructures and Nanotechnology, Institute of Chemistry, Chinese Academy of Sciences are solving the problem of the dissolution of polysulfide ions in high-specific energy lithium-sulfur batteries. An important breakthrough has been made in improving the cycle life of lithium-sulfur batteries. The research results were published in the recent J. Am. Chem. Soc. (2012, 134, 18510−18513), and were used by the American Chemical Society (ACS) Chemical & Engineering News as “Sustainable High Energy Battery†(High-Energy Battery Built To Last) was reviewed and reported. Lithium-sulfur battery refers to a type of metal lithium secondary battery that uses elemental sulfur (or sulfur-containing compound) as the positive electrode and metallic lithium as the negative electrode, and realizes the conversion between chemical energy and electrical energy through the chemical reaction between sulfur and lithium. Both elemental sulfur as the cathode material and metallic lithium as the anode material have a high theoretical specific capacity, so that the theoretical specific energy of the entire battery is as high as 2600Wh / kg, which is more than five times that of the existing lithium ion battery. However, due to the insulation properties of sulfur and its discharge product lithium sulfide (Li2S), as well as the shortcomings of a series of lithium polysulfide intermediate products formed during charge and discharge that are easily soluble in the electrolyte, the sulfur cathode activity of lithium-sulfur batteries is poor The utilization rate is low and the cycle performance is also very poor, which seriously affects the performance of the battery and its practical application. It is a difficult problem to be solved urgently. Researchers at the Key Laboratory of Molecular Nanostructures and Nanotechnology, Institute of Chemistry, Chinese Academy of Sciences realized that elemental sulfur mainly exists in the form of cyclic S8, and these soluble polysulfide ions (Li2S8, Li2S6, Li2S4, etc.) are mainly produced in S8 and S42- During the transition between. Together with the scientific researchers of the Bosch Asia-Pacific Science and Technology Research Center, starting from the design of sulfur molecular structure, they proposed the idea of ​​fundamentally solving the problem of dissolution of polysulfide ions by constructing small chain sulfur molecules (S2-4), and through the nanopore The spatial confinement effect of the system realizes the screening and stabilization of unconventional, metastable small sulfur molecules (Figure 1). They first synthesized a microporous carbon substrate with a specific pore size (0.5nm), and then loaded sulfur. Due to the limitation of the nanopore space, the conversion from S8 molecules to small sulfur molecules can be achieved during the introduction of sulfur, and an unconventional small sulfur molecule / carbon composite cathode material can be prepared. They collaborated with researchers from the Institute of Physics of the Chinese Academy of Sciences, using spherical aberration-corrected transmission electron microscopy and other advanced characterization methods and combined with theoretical calculations to prove that the form of sulfur in this nanopore is not the usual cyclic S8 molecule, but a small chain-like molecule. Sulfur molecule S2-4. The study found that this small chain sulfur molecule S2-4 exhibits a completely different electrochemical behavior from the cyclic S8 molecule during intercalation / delithiation, and no more soluble polysulfide ions (Li2S8 , Li2S6, Li2S4), so as to fundamentally solve the problem of poor cycle performance caused by the dissolution of polysulfide ions in traditional sulfur cathode materials. At the same time, because the size of sulfur particles has been reduced to the molecular level, the electrochemical activity of sulfur is significantly improved. The unconventional sulfur molecule / carbon composite cathode material based on the nano-channel confinement effect shows a high specific capacity, excellent cycle stability and high rate performance in lithium-sulfur batteries. The discharge capacity in the first lap calculated by the mass of sulfur reaches 1670mA h / g, which is close to the theoretical capacity of sulfur (1675mA h / g). After 200 cycles, there is still 1150mA h / g. The discovery of space-limited chain-like small sulfur molecules and their special electrochemical properties are of great significance for fundamentally solving the problem of dissolution of polysulfide ions in sulfur cathodes and developing high-performance lithium-sulfur batteries. Related results have applied for three PCT international patents. Figure 1 Schematic diagram of small sulfur molecules with restricted nanopores / positive materials for high-performance lithium-sulfur batteries. Fig. 2 (a) charge-discharge curve of small sulfur molecule / carbon composite cathode material, (b) cycle performance at 0.1C rate. Cosmetic Bag,Makeup Pouch,Travel Makeup Bag,Drawstring Makeup Bag Ningbo Happiness Stationery Industrial & Trading Co Ltd , https://www.bagshappiness.com